How Might the Internal Review Board Impact My Research on Caregivers
- Research
- Open up Admission
- Published:
Identifying treatment effects of an informal caregiver pedagogy intervention to increase days in the community and decrease caregiver distress: a machine-learning secondary analysis of subgroup effects in the HI-FIVES randomized clinical trial
Trials volume 21, Article number:189 (2020) Cite this article
Abstract
Background
Breezy caregivers study substantial burden and depressive symptoms which predict higher rates of patient institutionalization. While caregiver education interventions may reduce caregiver distress and subtract the utilise of long-term institutional care, show is mixed. Inconsistent findings across studies may be the result of reporting average treatment effects which exercise not account for how effects differ by participant characteristics. We apply a auto-learning approach to randomized clinical trial (RCT) information of the Helping Invested Family Members Amend Veteran'south Experiences Written report (Howdy-FIVES) intervention to explore how intervention effects vary by caregiver and patient characteristics.
Methods
We used model-based recursive segmentation models. Caregivers of customs-residing older developed US veterans with functional or cerebral impairment at a single VA Medical Center site were randomized to receive HI-FIVES (n = 118) vs. usual care (n = 123). The outcomes included cumulative days not in the community and caregiver depressive symptoms assessed at 12 months mail intervention. Potential moderating characteristics were: veteran age, caregiver age, caregiver ethnicity and race, human relationship satisfaction, caregiver burden, perceived fiscal strain, caregiver depressive symptoms, and patient risk score.
Results
The outcome of HI-FIVES on days non at dwelling was chastened by caregiver burden (p < 0.001); treatment furnishings were college for caregivers with a Zarit Burden Scale score ≤ 28. Caregivers with lower baseline Center for Epidemiologic Studies Depression Scale (CESD-10) scores (≤ 8) had slightly lower CESD-x scores at follow-up (p < 0.001).
Conclusions
Family caregiver educational activity interventions may be less benign for highly encumbered and distressed caregivers; these caregivers may require a more tailored arroyo that involves assessing caregiver needs and developing personalized approaches.
Trial registration
ClinicalTrials.gov, ID:NCT01777490. Registered on 28 January 2013.
Background
Maintaining aging adults at domicile is an important policy goal [1]. Informal caregiving, or providing unpaid treat a family unit member or friend, tin can substitute costly institutional-based long-term care [2, 3]. Withal, informal caregivers ofttimes report loftier levels of burden and depressive symptoms [4] which may pb to patient placement in institutional care [5]. Strengthening caregiver skills, support, and connection to wellness system resource, can reduce burden [6, 7] and psychological symptoms [8] and ameliorate the ability of caregivers to intendance for patients at home [9,10,xi]. Even so, systematic reviews of interventions for caregivers of multiple patient populations bear witness mixed results [12,13,14,xv,xvi]. By and large these systematic reviews were rigorously designed and included randomized controlled trial (RCT) pattern studies which lend credence to these results. Therefore, it is possible that inconsistencies in outcomes beyond studies are related to the limerick of the report samples. For example, within a written report sample the handling effect may be different for specific subgroups than for the overall sample [17]. A recent randomized clinical trial of a 9-session education intervention, Helping Invested Family Members Improve Veteran'due south Experiences Written report (Hullo-FIVES), for caregivers of veterans who were functionally impaired did not identify an average handling result on days in the community or caregiver depressive symptoms. The median of days not at home for participants randomized to HI-FIVES was 3 days vs. 3 days for control (i.e., usual care) participants while the mean of days not at dwelling house for Hullo-FIVES participants was 8.9 (SD = 13) vs. 6 (SD = 14.v) for control. At 12 months post baseline, caregivers in Hello-FIVES had a mean Center for Epidemiologic Studies Low Scale (CESD-ten) score of 8.2 (SD = 6.six) vs. vii.6 (SD = v.half-dozen) for the usual care group [18]. However, a subsequent written report using Hi-FIVES data examined the data for hypothesis-driven subgroup furnishings. This study institute that hospitalization risk moderated the effect of HI-FIVES; Veterans with a medium vs. loftier hospitalization take a chance spent more days at domicile as a result of the Hi-FIVES intervention [19].
However, it is possible that there remain systematically dissimilar outcomes among subgroups non identified a priori. Traditional statistical tests to identify effects amid multiple subgroups are underpowered and susceptible to multiple testing errors because they consider one gene at a time [xx]. Further, it is possible that combinations of characteristics, such as race/ethnicity and income, rather than single characteristics, requite ascent to these heterogeneous treatment effects—or differences in treatment consequence by subgroup [21]. To address these limitations, we employ automobile-learning methods for identifying heterogeneous subgroups to empathise which less discernible subgroups might benefit (or not) from the HI-FIVES intervention. Dissimilar traditional regression models which employ pre-specified structural hypotheses, machine learning seeks patterns in the data to identify important predictors and predictor interactions and are thus a preferable arroyo when the enquiry questions seek to observe associations rather than test a priori hypotheses. Specifically, we use model-based recursive partitioning methods to data from Howdy-FIVES [22] to examine the effect of predictors of handling furnishings across subgroups simultaneously, which is likely a more accurate portrayal of how private-level characteristics operate together to compound the benefits or risks of handling. In addition, this approach avoids multiple testing errors by building a decision tree through iteratively partitioning a space that comprises multiple covariates. In contrast, standard variable by variable interaction methods are but able to sectionalisation the space of ane covariate at a time which increases multiple testing error when analysts need to examine multiple potential moderators [23].
The objective of this report is to examine whether the boilerplate handling event of the HI-FIVES trial masked treatment effects amidst subgroups of the trial sample. This additional stride of mail-trial subgroup testing is of import for futurity interventions designed to target the needs of dyads who might receive beneficial effects [6, fourteen].
Methods
This study adheres to Consolidated Standards of Reporting Trials (CONSORT) guidelines.
Aim and study design
We applied machine-learning methods to conduct a mail-hoc analysis of the HI-FIVES trial data to explore whether treatment effects varied within subgroups of caregivers. Specifically, we tested for heterogeneous treatment effects of Hullo-FIVES, a RCT of a caregiver education intervention [18] on days non at abode and caregiver depressive symptoms amongst a sample of informal caregivers.
Participants
Breezy caregivers of patients who received a referral for Veteran Wellness Administration (VHA) home and customs-based services (HCBS) or geriatric clinics in the prior 6 months were identified through telephone contact with the patient (n = 3746). Note that individuals referred for obesity, diabetes, claret pressure level care or temporary care only were removed from the potential sample. Both the patient and caregiver had to qualify for the study. Ineligibility criteria for patients included (1) referral to nursing dwelling care or hospice in the past vi months, (ii) currently residing in an institution or hospital, (iii) identified as existence fully contained, (four) unable to communicate in English language, (v) having no phone number, (half dozen) no identified informal caregiver, and (vii) the presence of a behavioral flag in the medical records. Caregivers were ineligible if they were: (1) under historic period 18 years, (2) could non commit to attending four weekly group sessions, (3) currently participating in another caregiver written report, and (4) having v or more errors on the Short Portable Mental Status Questionnaire (SPMSQ). A full of 241 total dyads were consented and enrolled by the study research assistant [xviii]. Encounter Van Houtven, et al. for the Consort Diagram [18]. Dyads were stratified past patient cognitive status and whether the patient was a high health intendance utilizer and within the strata participants were randomly allocated 1:i to two arms (Hi-FIVES intervention vs. usual intendance) via a computer-generated randomization sequence. The study biostatistician conducted the randomization procedure. Loftier health care utilizer was defined every bit an individual with ii or more unique inpatient hospitalizations in the year prior to the almost contempo date of referral. Dyads in the treatment arm (n = 118) received a nine-session caregiver education intervention while dyads randomized to usual intendance (northward = 123) received routine services offered through the HCBS referral process. All caregivers received information about the Veterans Affairs (VA) Caregiver Back up Program (Public Law 111–163).
Intervention
Hi-FIVES comprised iii weekly individual phone training calls to the caregiver to improve behaviors related to medication management and four additional topics chosen by the caregiver [18, 24]. Topics included content such as rewards and frustrations of caregiving, clinical intendance, cocky-intendance, navigating the VA, planning for the time to come, and resources for caregivers. Following the telephone grooming calls, caregivers participated in iv weekly group didactics sessions lead by the interventionist and a VA caregiver support coordinator to address mutual issues facing caregivers of complex patients. Caregivers also received 2 private-level booster calls i and 2 months after completion of the group sessions.
Outcome measures
Our study considered two outcomes. The get-go result was the number of days the veteran was not at domicile (e.g., in emergency department (ED), infirmary or postal service-acute facility) during the 12 months following randomization; institutional hospice stays were not included every bit days not at home. This outcome was assessed using VA electronic health records and through phone verification with the caregiver to place hospitalizations that were not captured by VA wellness records. A 2.v-day decrease in the number of days not at dwelling during a 12-calendar month catamenia was hypothesized to be a clinically meaningful difference [25]. The 2nd outcome was caregiver depressive symptoms measured by the Center for Epidemiologic Studies Depression Scale (CESD-x) at 12 months postal service randomization [26]. A research assistant administered the CESD-ten at baseline during the in-person enrollment meeting at the Durham VA and at 12 months over the telephone. Patients were censored if they entered a residential nursing home or residential psychiatric inpatient unit (defined as a stay of > 60 days) or at expiry; for details nearly sample size adding, recruitment and attrition, unintended harms, and other aspects of study comport see [18].
Predictor measures
We assessed nine predictors that, based on existing evidence, were theorized to accept an of import moderating effect between the Hi-FIVES intervention and our outcomes of interest [27,28,29,30,31,32,33]. Predictors included caregiver age, caregiver ethnicity (Hispanic vs. not), caregiver race (White vs. not), caregiver burden, caregiver low, perceived financial difficulty (yes vs. no), relationship satisfaction, patient historic period, and patient medical complexity. These baseline measures were nerveless by the research banana from the caregivers at an in-person enrollment coming together. Subjective caregiver burden was measured using the continuous Zarit Burden Scale in which higher scores indicated higher subjective burden [34]. Relationship satisfaction was measured using the continuous caregiver relationship subscale of the Caregiver Appraisement Scale in which higher scores indicate more satisfaction (range 1–55) [35, 36]. Nosos risk scores, a continuous alphabetize of patient complexity (college score indicates more complexity [37]), takes into account the patients' diagnoses (ICD-9 codes), age, gender, and pharmacy records too every bit VA-specific items such as VA priority status and VA-computed costs. In the model for days not at home, we also included caregiver baseline depressive symptoms measured continuously using the CESD-10 [26]. This self-reported measure of depression is calculated by summing the scores of 10 items (the range is from 0 to 30 with scores of 10 or more indicating depressive symptoms).
The trial registration number is: NCT01777490.
Statistical analysis
Nosotros applied recursive partitioning methods to generalized linear models to construct decision copse by splitting nodes on the tree into daughter nodes to identify subgroups with substantially different furnishings from one another (https://cran.r-project.org/web/packages/partykit/vignettes/mob.pdf). Model-based recursive partitioning attempts to partition observations with respect to specific covariates and fit a local model in each cell of the partition. Score-based fluctuation tests the instability of the model'south parameters to make up one's mind the splits. Splitting ceases once the treatment-effect guess is homogenous within each cell; in other words, the algorithm estimates no further differences in treatment furnishings based on the remaining parameters that take not yet been partitioned.
Analytical steps for model-based recursive partitioning are: (ane) fit a parametric model to a dataset, (2) test for parameter instability over a set of segmentation variables, (3) if there is some overall parameter instability, split the model with respect to the variable associated with the highest instability, and (4) repeat the procedure in each of the daughter nodes [38].
Poisson (log link) and Gaussian distributions were used to model days not at dwelling house and caregiver depressive symptoms, respectively, at 12 months postal service randomization. Mean-centered stratification variables, patient cognitive and super-user condition, were included. For the days-non-at-home model we included an get-go for days observed (i.e., prior to censoring) and the stratification variables. The glmtree algorithm with default parameters in the partykit package in the R Statistical Environment was used. This algorithm preserves the randomized sample by examining combinations of interactions within treatment arm, which immune us to estimate handling furnishings under the assumption that observed and unobserved characteristics were similar across treatment and control arms. The models produced a glmtree for each outcome which we plotted and examined covariate remainder across handling arms within the identified subgroups using standardized mean differences (SMD); we used the convention of SMDs ≤ 0.ii to indicate an acceptable level of balance in small samples [39, 40].
We assessed the consistency of our results through x-fold cross-validation on our sample and past comparing our results with other automobile-learning algorithms that identify interactive effects. For the x-fold cantankerous-validation, the data was cut into 10 equally sized samples or folds; for each fold, the model was trained on 90% of the data and we assessed model fit—or how shut predictions are to the observed values—in the remaining 10% of the data. A single glmtree is produced for each fold so in addition to assessing model fit, nosotros besides examined the trees descriptively for variations in splits across folds compared with the tree built from the full dataset.
We applied 2 boosted methods to verify whether other auto-learning algorithms might identify like subgroups: mCART and random forest with interactions. The mCART approach was developed to improve balance among identified subgroups using RCT data; even when characteristics are counterbalanced on the total sample, imbalance in subgroups may drive faux detection of subgroup-specific effects [41]. mCART pair-matches treatment and control participants and estimates the treatment event inside each pair; a single tree is built to identify subgroups with differing treatment furnishings [41]. We also synthetic a random woods (randomForestSRC) that included all predictors and interactions betwixt the treatment and each predictor [42]. Nosotros so examined the 95% conviction intervals for the variable importance of the interaction terms [43, 44]. The mCART algorithm does not accommodate count models, and so we modeled the days-non-at-home outcome as a proportion of days non at home out of days in the study (count of days not at habitation/beginning). The random wood models exercise not rely on linearity assumptions and then our outcomes were specified the same mode equally they were in the glmtree algorithm. We also examined a binary indicator of any days not at home using a classification tree and the pair-matched algorithm.
Most variables had consummate data; however, CESD-10 at 12 months was missing for northward = 36 caregivers, nosos score was missing for north = 8 patients, and Zarit Burden score was missing for n = 2 caregivers. Virtually of the algorithms we used require complete data; therefore, we imputed the data for the variables in a higher place using adaptive tree imputations (randomSurvivalForest bundle) [43].
Results
Descriptive statistics
The total number of caregivers in the trial was 241; 118 in the intervention grouping and 123 in the command grouping. Patients on average were 73 (standard difference (SD) = xi.7) years sometime. The sample was primarily non-Hispanic just was comprised of over 50% non-Whites, primarily African-Americans. Patients in the sample prior to censoring had a mean of eight.viii (SD = 13.8) days not at dwelling over the 12 months post randomization; days not at home ranged from 0 to 80. Baseline caregiver Zarit Brunt scores averaged xviii.8 (SD = 9.7); caregiver baseline CESD-ten averaged 8.9 (SD = 5.9). Patients in this sample demonstrated substantial medical complexity; the mean nosos index was iii.4 (SD = 3.v). For additional details see Tabular array i.
Days-not-at-home result
The glmtree algorithm identified statistically pregnant differences in treatment effects betwixt caregivers with higher vs. lower Zarit Brunt scores (cutting-point identified by algorithm ≤ 28 vs. > 28; p = 0.01) (Fig. 1).
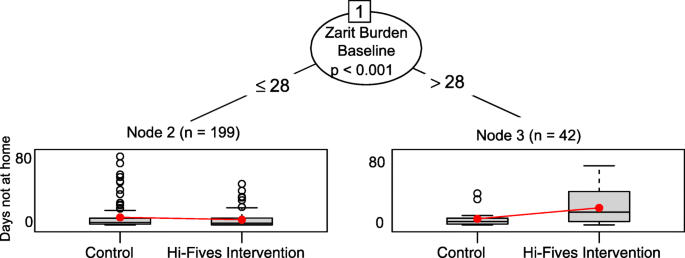
Glmtree algorithm for days not at home effect
Specifically, patients of caregivers with a Zarit Brunt score equal to or lower than 28 (n = 199) who participated in the How-do-you-do-FIVES intervention had a 40% increase in days at home compared with patients whose caregivers did not participate in Hullo-FIVES. For patients of caregivers with a Zarit Burden score greater than 28 (n = 42), participation in HI-FIVES was related to a 63% subtract in the number of days at domicile. Note that conclusion trees simply assess the statistical significance of differences in treatment effects between subgroups and exercise not provide confidence intervals for the event estimates within subgroups. As a sensitivity check, we ran a Poisson regression model, with a similar specification to the model used for the glmtree algorithm, within each subgroup. In both subgroups treatment effects were statistically significant. However, given that this is an exploratory written report and that our interest is in identifying subgroups, we do not focus on inferences virtually whether effect estimates represent a statistically pregnant difference betwixt the handling and control arms.
We identified several covariates that were not well-balanced across handling groups in the subgroups (Table 2). Among the loftier Zarit Burden score group, White race (vs. blackness), and human relationship satisfaction had SMDs college than 0.20. Among the low Zarit Burden score group, financial difficulty was imbalanced. Nine out of 10 trees produced by folds of the data identified a single separate on the Zarit Burden and showed similar trends in treatment effects amongst subgroups. One tree identified no subgroups. For the sensitivity analyses, the generalized linear trees plant no subgroups when we looked at the issue on any days in the community. mCART identified no interactions between study arm and whatsoever covariates using the proportion of days non at home out of all days in the study. The random forest with interactions algorithm identified statistically significant variable importance values for several interaction effects, including, in order of importance, the Zarit Burden score (highest variable importance), the baseline CESD-10 score, the nosos score, and patient age (see Table iii).
Caregiver depressive symptoms issue
The recursive sectionalization algorithm identified 1 dissever on depressive symptoms and produced two girl nodes (cut-point identified by algorithm ≤ 8 vs. > 8 on baseline CESD-10; p = 0.01) (Fig. 2). Caregivers with a baseline CESD-10 score of 8 or lower (n = 127) who participated in HI-FIVES experienced an average decrease in 0.06 points on the CESD-10 score at 12 months post intervention. Caregivers with a score greater than 8 (n = 114), on boilerplate, had a i.five higher CESD-x score at the terminate of follow-upwardly. Every bit a sensitivity check, nosotros also ran the linear regression models inside each subgroup, neither handling-effect gauge was statistically significant. However, our involvement is in identifying subgroups and not assessing within-subgroup treatment furnishings.
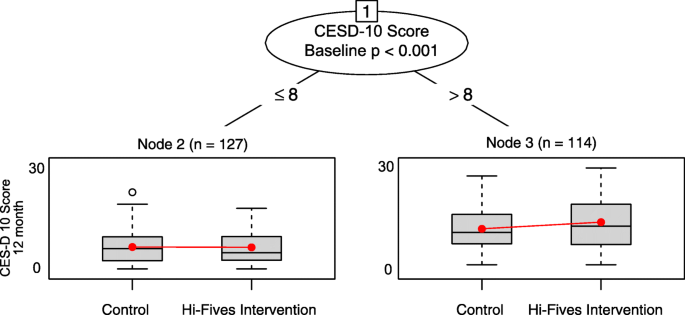
Glmtree algorithm for caregiver depressive symptoms consequence
We identified several covariates that were non well-counterbalanced across handling groups in the subgroups (Tabular array 2); among the higher baseline CESD-ten score, caregiver age, Zarit Burden score, and financial difficulty had SMDs greater than 0.twenty. Participants in the lower baseline CESD-D score subgroup were non well-balanced on patient age, Hispanic ethnicity, and perceived financial difficulty.
Across folds of the data, all ten glmtree algorithms identified a unmarried split on baseline CESD-x score and trends in treatment effects amid subgroups were similar. For the sensitivity analyses, mCART identified no interactions. The random forest algorithm identified interactions between study arm and baseline CESD score and Zarit Burden score (come across Tabular array 3).
Discussion
This report demonstrates how to utilise machine-learning algorithms with data from RCTs to explore potential subgroup furnishings that may be masked when trials examine outcomes as average treatment effects. We compare several algorithms, including a glmtree algorithm (master assay), mCART (sensitivity analysis), and random wood with interactions (sensitivity analysis). This is the first postal service-hoc analysis that uses machine learning to examine heterogeneous treatment effects of an intervention for informal caregivers.
The algorithm identified a cut-indicate of 28 on the Zarit Burden Scale—clinically significant burden is 18 and above [45]—therefore, a score of 28 and in a higher place (n = 42) represents a group of extremely distressed caregivers. For the CESD-10 consequence, the algorithm identified a cutting-signal of eight (CESD-10 > eight n = 114), which aligns with clinical standards for probable low [26]. For both outcomes, we only identified ane subgroup with differential treatment effects which suggests that these characteristics uniquely drove risk. Our sensitivity analyses likewise provide support for baseline caregiver brunt and depressive symptoms every bit potential moderators of the relationship between treatment and both of our outcomes at 12 months post intervention.
We did not test the statistical significance of the within-subgroup treatment-issue estimates in the model-based glmtree algorithms because we did not have a large plenty sample to train our model and so validate the findings in a test dataset. Notwithstanding, the treatment furnishings identified by the glmtree algorithm suggest that caregivers with higher baseline levels of burden and depression may not have been helped by the caregiver skills and education intervention. These findings must exist replicated, but information technology is possible that depression-intensity, brusque-term interventions are not enough to help highly distressed and encumbered caregivers. In fact, individualized or ane-on-1 interventions that target a specific outcome may be required make substantial improvements [sixteen].
Limitations and considerations
Our written report also highlights the challenges of applying machine learning to health services research, in general, and to post-hoc analyses of clinical trials, in particular [23, 46]. In that location are notable limitations both in the methods and in the programs available to implement the methods. Start, machine-learning methods do not require large sample sizes and are known to work well for datasets with many predictors relative to observations. Nonetheless, modest samples (n < 400) [47] may pose limitations because there are not plenty observations to train and exam algorithms and to produce fit statistics for the principal model. This is a major challenge for post-hoc analyses of trial data considering most intervention trials in wellness intendance take relatively pocket-size samples. The piece of work that has been done to engagement to utilize machine learning to trial data has taken advantage of big wellness trials [23, 46]. To address this challenge with our small sample we examined the consistency across folds of our dataset; our trees were consequent.
Another challenge related to small-scale sample size is that we were unable to generate measures of variability for subgroup level treatment-event estimates. The glmtree algorithm that we used provided a measure of statistical significance indicating whether or not at that place were differences in treatment-outcome sizes between subgroups and not whether the handling outcome itself was statistically significant within subgroups. While the model output provided a within-subgroup treatment-effect approximate, it did not provide a measure out of variability of the effect gauge. Therefore, we only report these estimates and not associated confidence intervals considering nosotros did not have a large enough sample to train the glmtree algorithm and run this algorithm on a validation dataset to generate standard errors for the estimates. Even so, the goal of our assay is to explore potential heterogeneous treatment effects and not to report treatment effects by subgroup.
Second, an inherent problem with single decision trees is that they tend to overfit the data [48]. In addition, simulation studies advise that characteristics within identified subgroups may non be balanced across treatment and command groups, fifty-fifty if characteristics are balance on the total sample, which could falsely induce subgroup identification [41]. Indeed our subgroups were not fully balanced on baseline covariates (Tabular array ii). To attempt to address the potential limitations of overfitting and poor residue, we examined the trees across folds of the data (described above) and ran several analyses to test the robustness of the results. First, we used the mCART approach—which balances on matched pairs and thus ensures that subgroups identified by the decision tree are counterbalanced. We also searched for interactive effectives using a random wood with interactions algorithm. While the mCART algorithm did not identify subgroups for the days-not-at-home event, the random forest model did identify an interaction between treatment and baseline CESD-10. mCART is inefficient for modest samples [41], which may explain why nosotros did non place any subgroups using this method for the days-non-at-home effect. Because of this, we too applied a virtual twin approach [49], which is not bounded by linearity assumptions; the results using this approach confirmed our results from the main analyses for both outcomes.
Statistical environments, including R and Python, offer the almost variety of auto-learning packages, even so package development in these environments is user-driven. Every bit machine learning is simply starting to be used for health services inquiry, many of the existing packages do not suit effect specifications commonly used in the field. mCART does not arrange count outcomes and, therefore, we modeled prevalence of days not at home using a linear model with normal distribution which would have been more likely to produce biased variance estimates; modeling the information using Poisson regression could accept led to more efficient and accurate estimates. Different outcomes specifications (i.eastward., count of days vs. proportion of days) may be some other reason why our sensitivity analyses did not identify subgroups.
Research implications
Nosotros attempted several approaches to limit the touch of these external limitations. Our goal was to identify subgroups with heterogeneous treatment effects to help time to come caregiver interventionists improve target their population. While we were unable to fully overcome these limitations, we offer a novel approach and considerations for other researchers who wish to conduct post-hoc trial analyses. For researchers who are designing interventions for highly burdened and distressed caregivers, a tailored, more than intensive intervention that involves assessing caregiver needs and developing personalized approaches may exist warranted. However, caregivers with lower levels of brunt and depression may benefit from a group and telephone-based skills training program, such every bit HI-FIVES.
Conclusions
Using model-based recursive sectionalization methods to conduct a post-hoc analysis of subgroup furnishings of the HI-FIVES intervention, we institute potential testify for heterogeneous treatment effects. In general, use of these methods can be constrained past limitations that are mutual in RCTs of clinical interventions, including small sample sizes and outcomes that do not run across the distributional assumptions of machine-learning algorithms in existing software programs. We present a process for applying these methods using data with such limitations and suggest various sensitivity analyses and robustness checks. Further, we demonstrate how our results can be used for hypothesis generation every bit opposed to inference about subgroup effects.
Availability of information and materials
The datasets generated and/or analyzed during the electric current study are not publicly available because private privacy may be comprised and nosotros practise not have permission to share this private data, only analytical models and code are available from the corresponding writer on reasonable request.
Abbreviations
- CESD-x:
-
Center for Epidemiologic Studies Low Scale
- ED:
-
Emergency department
- HCBS:
-
Home and community-based services
- HI-FIVES:
-
Helping Invested Family Members Improve Veteran's Experiences Study
- SD:
-
Standard divergence
- SMD:
-
Standardized mean differences
- SPMSQ:
-
Mental Status Questionnaire
- VHA:
-
Veteran Health Administration
References
-
Olmstead v. L.C., 527 U.Due south. 581. 1999.
-
Van Houtven CH, Norton EC. Informal intendance and health intendance use of older adults. J Health Econ. 2004;23(half dozen):1159–eighty.
-
Van Houtven CH, Norton EC. Informal care and Medicare expenditures: testing for heterogeneous treatment effects. J Health Econ. 2008;27(1):134–56.
-
Epstein-Lubow Thousand, Davis JD, Miller IW, Tremont G. Persisting burden predicts depressive symptoms in dementia caregivers. J Geriatr Psychiatry Neurol. 2008;21(3):198–203.
-
Toot S, Swinson T, Devine G, Challis D, Orrell Thou. Causes of nursing home placement for older people with dementia: a systematic review and meta-analysis. Int Psychogeriatr. 2017;29(ii):195–208.
-
Gitlin LN, Belle SH, Burgio LD, Czaja SJ, Mahoney D, Gallagher-Thompson D, et al. Issue of multicomponent interventions on caregiver burden and depression: the Attain multisite initiative at 6-month follow-up. Psychol Crumbling. 2003;18(3):361–74.
-
Adelman RD, Tmanova LL, Delgado D, Dion S, Lachs MS. Caregiver burden: a clinical review. JAMA. 2014;311(x):1052–60.
-
Belle SH, Burgio L, Burns R, Coon D, Czaja SJ, Gallagher-Thompson D, et al. Enhancing the quality of life of dementia caregivers from different ethnic or racial groups: a randomized, controlled trial. Ann Intern Med. 2006;145(x):727–38.
-
Gerdner LA, Buckwalter KC, Reed D. Touch of a psychoeducational intervention on caregiver response to behavioral bug. Nurs Res. 2002;51(six):363–74.
-
Gitlin LN, Winter L, Corcoran M, Dennis MP, Schinfeld S, Hauck WW. Effects of the abode environmental skill-building program on the caregiver-care recipient dyad: half-dozen-calendar month outcomes from the Philadelphia Accomplish Initiative. Gerontologist. 2003;43(four):532–46.
-
Hepburn Chiliad, Lewis M, Tornatore J, Sherman CW, Bremer KL. The Savvy Caregiver Program: the demonstrated effectiveness of a transportable dementia caregiver psychoeducation program. J Gerontol Nurs. 2007;33(3):30–vi.
-
Lins S, Hayder-Beichel D, Rucker 1000, Motschall E, Antes G, Meyer One thousand, et al. Efficacy and experiences of telephone counselling for breezy carers of people with dementia. Cochrane Database Syst Rev. 2014;(nine):CD009126. https://doi.org/10.1002/14651858.CD009126.pub2.
-
Thompson CA, Spilsbury Thousand, Hall J, Birks Y, Barnes C, Adamson J. Systematic review of information and back up interventions for caregivers of people with dementia. BMC Geriatr. 2007;7:18.
-
Goy East, Kansagar D, Freeman M. A systematic testify review of interventions for non-professional caregivers of individuals with dementia. Washington, DC: Section of Veteran Affairs; 2010.
-
McGriffin JA, Meis L, Carlyle M, Greer North, Jensen A, Macdonald R, et al. Effectiveness of family and caregiver interventions on patient outcomes among adults with cancer or memory-related disorders: a systematic review. Washington, DC: Department of Veteran Diplomacy; 2013.
-
Shepherd-Banigan M, McDuffie JR, Shapiro A, Brancu M, Sperber N, Mehta NN, et al. Interventions to support caregivers or families of patients with TBI, PTSD, or polytrauma: a systematic review. Washington, DC: Department of Veteran Diplomacy, Veterans Health Assistants; 2017. Report No.: 09–001
-
Greenfield Due south, Kravitz R, Duan Northward, Kaplan SH. Heterogeneity of handling furnishings: implications for guidelines, payment, and quality assessment. Am J Med. 2007;120(iv Suppl 1):S3–ix.
-
Van Houtven CH, Smith VA, Lindquist J, Chapman JG, Hendrix C, Hastings SN, et al. Family caregiver skills training to improve experiences of intendance: a randomized clinical trial. J Gen Intern Med. 2019; In printing.
-
Cary MP, Smith VA, Shepherd-Banigan Chiliad, Lindquist JH, Chapman JL, Hastings SN, et al. Moderators of treatment outcomes from family caregiver skills preparation: secondary assay of a randomized controlled trial. OBM Geriatr. 2019;3(2):xiv. https://doi.org/10.21926/obm.geriatr.1902049.
-
Kraemer HC, Frank E, Kupfer DJ. Moderators of treatment outcomes: clinical, inquiry, and policy importance. JAMA. 2006;296(10):1286–ix.
-
VanderWeele TJ, Knol MJ. Estimation of subgroup analyses in randomized trials: heterogeneity versus secondary interventions. Ann Intern Med. 2011;154(10):680–3.
-
Sies A, Van Mechelen I. Comparing four methods for estimating Tree-based treatment regimes. Int J Biostat. 2017;13(ane). https://doi.org/10.1515/ijb-2016-0068.
-
Baum A, Scarpa J, Bruzelius E, Tamler R, Basu Due south, Faghmous J. Targeting weight loss interventions to reduce cardiovascular complications of blazon 2 diabetes: a motorcar learning-based post-hoc assay of heterogeneous treatment effects in the Look AHEAD trial. Lancet Diabetes Endocrinol. 2017;5(10):808–xv.
-
Van Houtven CH, Oddone EZ, Hastings SN, Hendrix C, Olsen M, Neelon B, et al. Helping Invested Families Better Veterans' Experiences Study (Hullo-FIVES): written report design and methodology. Contemp Clin Trials. 2014;38(two):260–9.
-
Ariti CA, Cleland JG, Pocock SJ, Pfeffer MA, Swedberg K, Granger CB, et al. Days alive and out of infirmary and the patient journey in patients with heart failure: Insights from the Candesartan in Centre failure: Assessment of Reduction in Bloodshed and morbidity (Amuse) program. Am Heart J. 2011;162(5):900–6.
-
Andreson E, Malmgren J, Carter W, Patrick D. Screening for low in well older adults: evaluation of a short form of the CES D. Am J Prev Med. 1994;4:77–84.
-
Gallagher D, Ni Mhaolain A, Crosby Fifty, Ryan D, Lacey L, Coen RF, et al. Determinants of the desire to institutionalize in Alzheimer's caregivers. Am J Alzheimers Dis Other Bewilder. 2011;26(iii):205–11.
-
Wolff JL, Mulcahy J, Roth DL, Cenzer IS, Kasper JD, Huang J, et al. Long-term nursing home entry: a prognostic model for older adults with a family or unpaid caregiver. J Am Geriatr Soc. 2018;66(x):1887–94.
-
Spitznagel MB, Tremont G, Davis JD, Foster SM. Psychosocial predictors of dementia caregiver desire to institutionalize: caregiver, care recipient, and family relationship factors. J Geriatr Psychiatry Neurol. 2006;nineteen(1):16–20.
-
Vandepitte Southward, Putman M, Van Den Noortgate N, Verhaeghe South, Mormont E, Van Wilder L, et al. Factors associated with the caregivers' desire to Institutionalize persons with dementia: a cross-sectional study. Dement Geriatr Cogn Disord. 2018;46(5–6):298–309.
-
Pruchno RA, Michaels JE, Potashnik SL. Predictors of institutionalization amongst Alzheimer disease victims with caregiving spouses. J Gerontol. 1990;45(half-dozen):S259–66.
-
Schulz R, Williamson GM. A two-year longitudinal written report of low among Alzheimer'southward caregivers. Psychol Aging. 1991;6(4):569–78.
-
Covinsky KE, Newcomer R, Fox P, Wood J, Sands L, Dane K, et al. Patient and caregiver characteristics associated with depression in caregivers of patients with dementia. J Gen Intern Med. 2003;xviii(12):1006–14.
-
Zarit SH, Reever KE, Bach-Peterson J. Relatives of the impaired elderly: correlates of feelings of burden. Gerontologist. 1980;xx(6):649–55.
-
Lawton MP, Kleban MH, Moss Chiliad, Rovine M, Glicksman A. Measuring caregiving appraisal. J Gerontol. 1989;44(3):P61–71.
-
Struchen MA, Atchison TB, Roebuck TM, Caroselli JS, Sander AM. A multidimensional measure of caregiving appraisal: validation of the Caregiver Appraisement Calibration in traumatic brain injury. J Head Trauma Rehabil. 2002;17(2):132–54.
-
Wagner T, Upadhyay A, Cowgill East, Sterfos T, Moran E, Asch Due south, et al. Risk adjustment tools for learning health systems: a comparison of DxCG and CMS-HCC V21. Health Serv Res. 2016;51(5):2002–19. In printing.
-
Zeileis A, Hothorn T, Hornik K. Model-based recursive partitioning. J Comput Graphical Stat. 2008;17(2):492–514.
-
Yang D, Dalton J, editors. A unified approach to measuring the effect size between two groups using SAS. SAS Global Forum 2012:335–2012. SAS.
-
Austin PC. Residue diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28(25):3083–107.
-
Rigdon J, Baiocchi M, Basu S. Preventing fake discovery of heterogeneous treatment effect subgroups in randomized trials. Trials. 2018;19(1):382.
-
Bien J, Taylor J, Tibshirani R. A lasso for hierarchical interactions. Ann Stat. 2013;41(three):1111–41.
-
Ishwaran H, Kogalur UB. Consistency of random survival forests. Stat Probab Lett. 2010;lxxx(13–14):1056–64.
-
Ishwaran H, Lu M. Standard errors and conviction intervals for variable importance in random forest regression, classification, and survival. Stat Med. 2019;38(4):558–82.
-
Zarit SH, Orr NK, Zarit JM. The hidden victims of Alzheimer'southward disease: families under stress. New York: New York Academy Press; 1985.
-
Basu Due south, Raghavan S, Wexler DJ, Berkowitz SA. Characteristics associated with decreased or increased mortality gamble from glycemic therapy among patients with type 2 diabetes and high cardiovascular risk: machine learning analysis of the Accordance Trial. Diabetes Care. 2018;41(3):604–12.
-
Dusseldorp Eastward, Van Mechelen I. Qualitative interaction trees: a tool to identify qualitative handling-subgroup interactions. Stat Med. 2014;33(2):219–37.
-
Breiman L, Friedman JH, Olshen RA, Stone CJ. Classification and regression copse. Monterey: Wadsworth & Brooks/Cole Advanced Books; 1984.
-
Foster JC, Taylor JM, Ruberg SJ. Subgroup identification from randomized clinical trial information. Stat Med. 2011;thirty(24):2867–lxxx.
Acknowledgements
This manuscript was supported by the Department of Veterans Diplomacy, Veterans Health Administration, Function of Inquiry and Development, Health Services Research and Development Service (IIR 11-345). Additional back up comes from the Durham Center of Innovation to Accelerate Discovery and Practice Transformation (ADAPT) (CIN 13-410) and the Geriatrics Research Didactics and Clinical Eye at the Durham VA Health Care Arrangement. Dr. Shepherd-Banigan was funded, in role, by the VA OAA HSR&D Postdoctoral Fellowship Program (TPH 21-000). Dr. Cary is funded, in part, by 5KL2TR002554-02 and an University Wellness System Sciences Fellowship. We want to thank Dr. Joseph Rigdon for sending u.s. the code for the mCART packet which has not yet been published in R.
Funding
This manuscript was supported by the Section of Veterans Diplomacy, Veterans Wellness Assistants, Office of Research and Evolution, Health Services Research and Evolution Service (IIR 11–345). Additional support comes from the Durham Eye of Innovation to Accelerate Discovery and Practice Transformation (ADAPT) (CIN 13–410) and the Geriatrics Research Education and Clinical Center.at the Durham VA Health Care Arrangement. Dr. Shepherd-Banigan was funded, in function, by the VA OAA HSR&D Postdoctoral Fellowship Program (TPH 21–000). Dr. Cary is funded, in function, by 5KL2TR002554–02 and an Academy Wellness System Sciences Fellowship.
The funders had no role in the blueprint of the report and the collection, analysis or interpretation of the data or in writing the manuscript.
Writer data
Affiliations
Contributions
MSB conceptualized the idea, conducted the analyses, and wrote the paper. VAS made substantial contributions to the design of the work and data assay and interpretation; she also essentially revised the paper. JHL provided substantial contribution to the conquering of the data and interpretation of results; she also substantially revised the newspaper. MPC provided substantial contributions to the conception of the work and substantially revised the paper. KEMM provided substantial contributions to the formulation of the work and substantially revised the paper. JGC provided substantial contributions to the acquisition of the data and revisions to the paper. CHVH provided substantial contributions to the conception of the piece of work and acquisition of the information and she essentially revised the paper. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Human subjects' approval was provided by the Durham VA Institutional Review Board.
Informed consent was obtained from all study participants; human subjects were enrolled and completed and signed informed consent documentation.
Consent for publication
Non applicative.
Competing interests
The authors declare that they have no competing interests.
Additional data
Publisher'south Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Admission This commodity is distributed nether the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted utilize, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Artistic Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zip/ane.0/) applies to the data made bachelor in this article, unless otherwise stated.
Reprints and Permissions
About this article
Cite this article
Shepherd-Banigan, M., Smith, 5.A., Lindquist, J.H. et al. Identifying treatment effects of an informal caregiver education intervention to increase days in the community and decrease caregiver distress: a machine-learning secondary assay of subgroup effects in the Hi-FIVES randomized clinical trial. Trials 21, 189 (2020). https://doi.org/10.1186/s13063-020-4113-x
-
Received:
-
Accepted:
-
Published:
-
DOI : https://doi.org/10.1186/s13063-020-4113-ten
Keywords
- Family caregiver intervention
- Institutionalization
- Caregiver depression
- Clinical trial
- Heterogeneous treatment effects
Source: https://trialsjournal.biomedcentral.com/articles/10.1186/s13063-020-4113-x
0 Response to "How Might the Internal Review Board Impact My Research on Caregivers"
إرسال تعليق